Introduction: The RAS superfamily of small GTPases mediate hallmarks of cancer cell proliferation, viability, migration, invasion and metastasis. Several GTPases such as RAL, RHO and RAC are required for RAS to induce malignant transformation. RAS post-translational modification (PTM) by farnesyltransferase (FT) as well as RAL, RHO and RAC PTM by geranylgeranyltransferase 1 (GGT-1) are critical for the membrane localization and oncogenicity of these GTPases. Mutations in HRAS, NRAS, KRAS and RHOA are prevalent in solid tumors as well as T-cell lymphomas (TCL) such as peripheral T-cell lymphoma (PTCL), angioimmunoblastic T-cell lymphoma (AITL), PTCL-not otherwise specified and cutaneous T-cell lymphoma (CTCL). PTX-100 is a small molecule inhibitor of GGT-1 that disrupts the oncogenic activity by inhibiting RAL, RHO and RAC PTM.
Methods: This ongoing phase 1 study (NCT03900442) in 2 Australian sites, consists of a 3+3 dose escalation with an expansion phase. The aim is to evaluate the pharmacodynamic (PD), pharmacokinetics (PK), preliminary efficacy and safety of 500, 1000 and 2000 mg/m 2 PTX-100 in patients (pts) with advanced malignancies. PTX-100 is administered by IV infusion over 60 minutes days 1 to 5 of a 14 day cycle for 4 cycles. Disease response is assessed via CT, MRI or PET-CT or per SOC after 4 cycles. Pts with a complete response (CR), partial response (PR) or stable disease (SD) may be eligible for continued treatment with PTX 100, dosed in 21 day cycles with response assessment every 3 months.
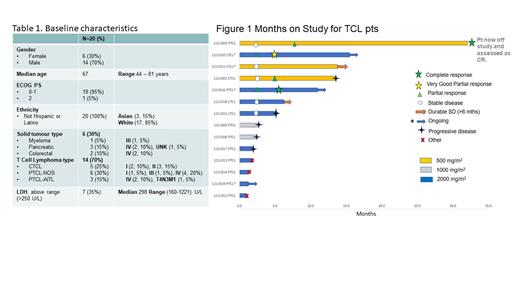
Results: The first pt was enrolled in October 2019 and enrollment is nearly completed in the expansion cohort. The data cutoff was 10 th July 2023. Ten pts were enrolled into Part 1 with 3 dosed at 500 mg/m 2, 3 at 1000 mg/m 2 and 4 at 2000 mg/m 2. The expansion cohort at 2000 mg/m 2 began in May 2022 and there have been 10/15 enrolled to date. Positive long-term responses (32 and 13.8 months) were observed for 2 TCL pts in Part 1 so the decision was made to enroll TCL pts into the expansion cohort. The median age was 67 years (44 to 81), the median number of prior lines was 3 (1-6). The TCL subtypes were PCTL NOS (n=6), AITL (n=3), and CTCL (n=5).
Of the 14 enrolled TCL pts, 10 pts had response assessments after 4 cycles, with an overall response rate (ORR) of 40% (4pts), including 10% (1 pt) CR, 30% (3pts) PR. Two CTCL pts (20%) had durable SD >6 months, contributing to a 60% Disease Control Rate. The median Progression Free Survival (PFS) for all TCL pts was 5.3 mos, CTCL pts 13.6 mos and PTCL pts 2.5 mos. No responses were observed in the 5 solid tumor pts.
There were 23 Grade ≥3 adverse events in 8 pts including laboratory neutropenia in 2 pts with 1 deemed possibly related. Neutrophil count decreased in 2 pts with 1 deemed possibly related and the platelet count decreased in 2 pts with 1 deemed possibly related. Grade 1-2 adverse events deemed possibly related included, nausea reported in 4 pts and diarrhea in 3 pts.
For the PD studies, we determined the effects of PTX-100 on the geranylgeranylation (GG) of the small GTPase RAP1 in peripheral blood monocytes (PBMCs) isolated from pts on cycle 1, day 1 (prior to treatment) and subsequently on days 2, 3, 4, 5, and 8. PTX-100 inhibited GG of RAP1A as early as 1 day after treatment, and with the majority of the pts, the inhibition of RAP1A GG persisted three days after the last dose of PTX-100. Inhibition of RAP1 GG was observed with all doses including the lowest dose of 500 mg/m 2.
PK analysis is underway at the time of abstract preparation and the PK parameters will be presented at the meeting.
Conclusions: Preliminary data from this phase 1 dose escalation study of PTX-100 indicates promising safety profile in this difficult to treat pt population. PTX-100 demonstrated clinical activity in TCL with an ORR of 40%, and a median PFS for all TCL pts of 5.3 months. PD studies demonstrated that the GGT-1 target was engaged with sustained inhibition of RAP1 GG.
Disclosures
Chew:Prescient Therapeutics Ltd: Consultancy, Current equity holder in publicly-traded company. Grant:Prescient Therapeutics: Research Funding. Sebti:Prescient Therapeutics: Consultancy, Current equity holder in publicly-traded company, Other: Scientific Founder, Patents & Royalties. Prince:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal